Rise-time Measurement Instrument Stig
RISE TIME – A Key Attribute of Dry-Powder Device Performance and Testing
Compendial methods of testing DPIs call for an abrupt start to the air flow through the inhaler and into the cascade impactor. This procedure is logical because real users do the same. It is recognized, of course, that this “in vitro” compendial testing cannot truly simulate real patient use. However, product developers will maximize the value of this quality control test if the drug product exits the DPI in a time frame similar to that of real users. It is the RISE TIME measurement accomplished by STIG that enables this evaluation to be made. This evaluation can lead to productive adjustment to devices still under development and can lead to better designed and controlled quality testing of registered drug products.
Experimental and computational studies of the RISE TIME in compendial testing are continuing to elucidate the relationship of device characteristics and the test system itself. These studies are critical because the device and the cascade impactor and connecting plumbing components together comprise a unique fluid mechanical system, one that does not exist in real patient use. The STIG instrument offered by FIA enables real-time experimental investigation of these important fluid flow dynamics, dynamics that can transpire in under 100 millisecond.
Now, more than ever, FIA’s NEW Laminar Flow STIG enables millisecond response times, with no thermal lag, giving users unprecented accuracy in making this measurement.
A training programme is offered together with the instrument, which helps the user to understand its usage and our experts will help you get started understanding how to setup your measurement.
Trade Item Number: Rise-time Measurement Instrument Stig
Trade Item Number: Training and IQ Rise Time Equipment Stig
Key features of Stig:
- Rise-time measurement 0.1-1 s using a laminar flow element
- Average rise-time from a series of measurements
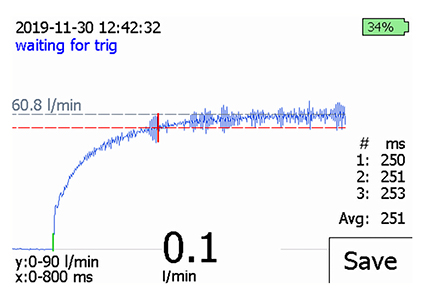
- Touch-screen which displays a graph of flow vs. time, rise-time and the final flow
- Printed records of the measurement with optional printer
- Rise-time profile saved to USB-memory
- Date and time
- Battery powered
- IQ/OQ and quality certificate for the regulated industry
- Training program and specialist support available
Rise-time Measurement Instrument Stig
It is important that the rise-time is known and under control when testing inhalation devices. Russell-Graham and colleagues1 showed that the fine particle dose increases as the rise time decreases. Previous and subsequent theoretical analyses point out reasons for this effect.2,3 For established products, then, quality control (QC) testing requires knowing that the rise-time remains in a specified range…now possible with Stig. For GMP QC Stig can be locked to acquire and present the rise-time according to a defined and validated method.
Since patients do generate different inhalation air-flow profiles, it is also important during the development of a new drug product to adjust the prototype devices to exhibit a sensible rise-time, close to what will take place in patient use. This mindset is equally important for DPIs and for breath-actuated MDI devices.
Stig also makes possible the recording of air-flow profiles so that they can be reproduced on a breathing simulator (such as FIA’s equipment F-SIG 6300) for studying nebulizers. Versatile, user-friendly, best-in-class – that is the Stig from AB FIA.
Download the pdf Rise-time Measurement Stig

References
- Russell-Graham, D., A. Cooper, B. Stobbs, E. McAulay, H. Bogard, V. Heith, E. Monsallier, “Further Evaluation of the Fast-Screening Impactor for
Determining Fine-Particle Fraction of Dry Powder Inhalers,” Drug Delivery to the Lung, December 8 to 10, 2010, Edinburgh, Scotland. - Roberts, D. L., M. Chiruta, “Transient Impactor Behavior during the Testing of Dry-Powder Inhalers via Compendial Methods,” Drug Delivery
to the Lung 18, The Aerosol Society, Edinburgh, Scotland, December 13-14, 2007. - Versteeg, H., P. Zhao, C. Blatchford, M. Copley, D. L. Roberts, J. P. Mitchell, “A Computational Fluid Dynamics (CFD) Model of the Start-Up Kinetics
of the Andersen Cascade Impactor (ACI),” Drug Delivery to the Lung, Aerosol Society, Edinburgh, Scotland, December 9-11, 2015; pages 18-21.
